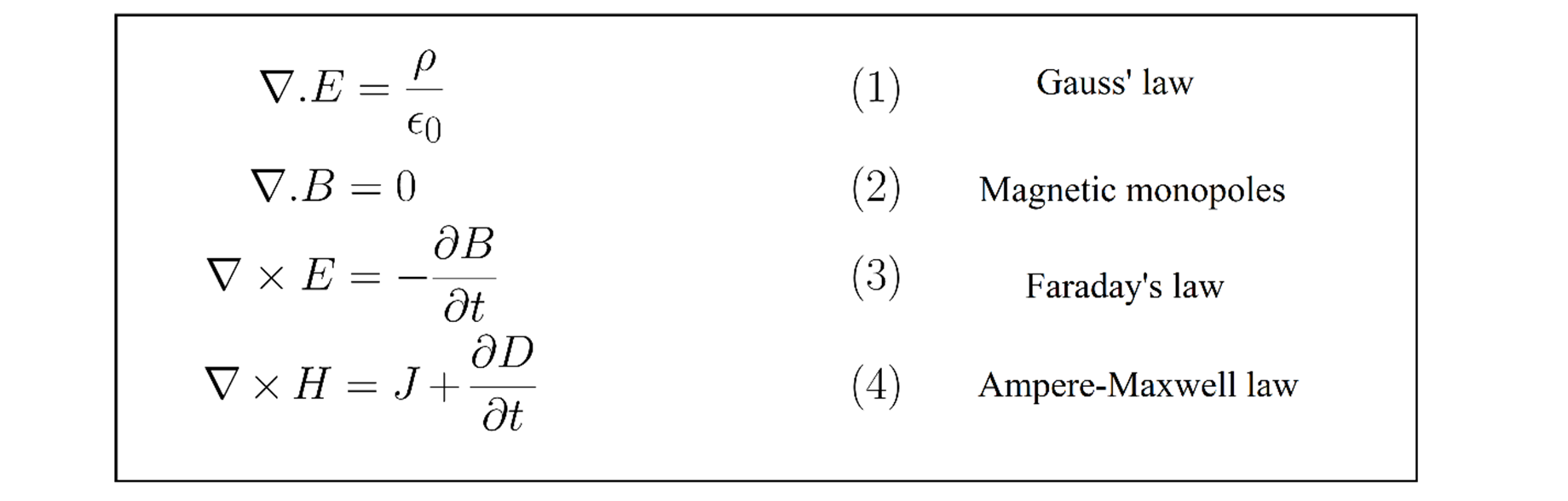
What are the 4 Maxwell equations
The four Maxwell equations, corresponding to the four statements above, are: (1) div D = ρ, (2) div B = 0, (3) curl E = -dB/dt, and (4) curl H = dD/dt + J.
Why are there 4 Maxwell equations
Maxwell didn't invent all these equations, but rather he combined the four equations made by Gauss (also Coulomb), Faraday, and Ampere. But Maxwell added one piece of information into Ampere's law (the 4th equation) – Displacement Current, which makes the equation complete.
What is Maxwell’s 4th equation
∮→B⋅d→ℓ=μ0(I+ddt(ε0∫→E⋅d→A)). This is Maxwell's fourth equation. Notice that in the case of the wire, either the current in the wire, or the increasing electric field, contribute on the right hand side, depending on whether we have the surface simply cutting through the wire, or positioned between the plates.
What are the application of 4th Maxwell’s equation
Answer: The main applications of Maxwell equations are we can calculate static electric field in a vacuum, we can calculate static magnetic field in a vacuum, it gives us the idea about electric circuits.
What are the four most common Maxwell relations
Thermodynamic Potentials
| Thermodynamic Potentials | The Derived Derivational Form | The Natural Variables |
|---|---|---|
| Internal Energy depicted by U | dU = TdS − PdV | S and V |
| Enthalpy depicted by H | dH = TdS + VdP | S and P |
| Helmholtz Free Energy as depicted by F | dF = −PdV − SdT | V and T |
| Gibbs Free Energy as depicted by Gd | G = VdP − SdT | P and T |
What are the four Maxwell’s thermodynamic relations
Common forms of Maxwell's relations
| Function | Differential | Natural variables |
|---|---|---|
| U | dU = TdS – PdV | S, V |
| H | dH = TdS + VdP | S, P |
| F | dF = -PdV – SdT | V, T |
| G | dG = VdP – SdT | P, T |
How many Maxwell equations are there
four
Although there are just four today, Maxwell actually derived 20 equations in 1865. Later, Oliver Heaviside simplified them considerably. Using vector notation, he realised that 12 of the equations could be reduced to four – the four equations we see today.
Are all four Maxwell equations independent
According to this reasoning, the electromagnetic theory is not based on four independent Maxwell equations but rather on three independent equations only; namely, the Faraday-Henry law (1c), the Ampère–Maxwell law (1d), and the principle of conservation of charge (2).
What are the 4 types of thermodynamic systems
The four types of thermodynamic process are isobaric, isochoric, isothermal and adiabatic.
What are the 4 laws of thermodynamics called
And all other forms of energy and consequently all forms of energy that relate to one another. Well the four laws of thermodynamics. Work to define the foundational.
What are 4th thermodynamics laws
4. 'Fourth law of thermodynamics': the dissipative component of evolution is in a direction of steepest entropy ascent.
What are the 4 quantities used to express thermodynamics
The fundamental thermodynamic equations describe the thermodynamic quantities U, H, G, and A in terms of their natural variables. The term "natural variable" simply denotes a variable that is one of the convenient variables to describe U, H, G, or A.
Who discovered the 4 laws of thermodynamics
What are the laws of thermodynamics The first and second laws were formally stated in works by German physicist Rudolf Clausius and Scottish physicist William Thomson about 1860. The third law was developed by German chemist Walther Nernst from 1906 to 1912.
What are the 3 laws of thermodynamics
1st Law of Thermodynamics – Energy cannot be created or destroyed. 2nd Law of Thermodynamics – For a spontaneous process, the entropy of the universe increases. 3rd Law of Thermodynamics – A perfect crystal at zero Kelvin has zero entropy.
What is the 5th rule of thermodynamics
A central component of Thomas Kuhn's philosophy of measurement is what he calls the fifth law of thermodynamics. According to this “law,” there will always be discrepancies between experimental results and scientists' prior expectations, whether those expectations arise from theory or from other experimental data.
What is 4 law of thermodynamics in chemistry
There are four laws of thermodynamics. They talk about temperature, heat, work, and entropy. They are used in thermodynamics and other sciences, for example chemistry.
Is there a fourth law of thermodynamics
'Fourth law of thermodynamics': the dissipative component of evolution is in a direction of steepest entropy ascent.
What is 4th law of thermodynamics
4. 'Fourth law of thermodynamics': the dissipative component of evolution is in a direction of steepest entropy ascent.
What is 3 state third law of thermodynamics
The third law of thermodynamics states that the entropy of a system at absolute zero is a well-defined constant. This is because a system at zero temperature exists in its ground state, so that its entropy is determined only by the degeneracy of the ground state.
What are the 0th 1st 2nd and 3rd laws of thermodynamics
1st Law of Thermodynamics – Energy cannot be created or destroyed. 2nd Law of Thermodynamics – For a spontaneous process, the entropy of the universe increases. 3rd Law of Thermodynamics – A perfect crystal at zero Kelvin has zero entropy.
What is 4 law of thermodynamics
Four general rules of thermodynamic modelling reveal four laws of Nature: (1) when the system is well separated from its environment, its energy must be defined for all states and must emerge as an additive, exchangeable, and conserved property; (2a) when the system is uncorrelated from any other system, its entropy …
Is there a 4th law of thermodynamics
'Fourth law of thermodynamics': the dissipative component of evolution is in a direction of steepest entropy ascent.
What are the 4 thermodynamic processes
Types of Thermodynamic ProcessesIsobaric process in which the pressure (P) is kept constant (ΔP =0).Isochoric process in which the volume (V) is kept constant (ΔV =0).Isothermal process in which the temperature (T) is kept constant (ΔT =0).Adiabatic process in which the heat transfer is zero (Q=0).
What are the 4 stages of thermodynamics
David has taught Honors Physics, AP Physics, IB Physics and general science courses. He has a Masters in Education, and a Bachelors in Physics.
What is the 0 3 law of thermodynamics
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other.