What is the FDA of India
FDA opened the India Office in New Delhi in 2008, to ensure that food and medical products exported from India to the U.S. are safe, are of good quality, and are effective.
What is the full form of FDA in food industry
The FDA (U.S. Food and Drug Administration) is an agency within the U.S. Department of Health and Human Services (HHS) that oversees the manufacturing and distribution of food, pharmaceuticals, medical devices, tobacco and other consumer products and veterinary medicine.
What is the full form of FDA approval
The Food and Drug Administration is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of our nation's food supply, cosmetics, and products that emit radiation.
Does FDA apply in India
Established in November 2008, the Office of Global Policy and Strategy's (OGPS) India Office (INO) serves as the lead FDA on-site presence in New Delhi, India. The India Office addresses operational and policy matters concerning FDA-regulated products in collaboration with the Government of India counterparts.
Who approves drugs in India
The Central Drugs Standard Control Organisation(CDSCO)under Directorate General of Health Services,Ministry of Health & Family Welfare,Government of India is the National Regulatory Authority (NRA) of India.
Does food have to be FDA approved
Food facilities do not have to obtain any type of certification or approval before distributing products in the United States. Food facilities are required to register with FDA, but being registered with FDA does not indicate FDA approval of the facility or its products. New food additives do require FDA approval.
How can I get FDA license in India
The License process described below:Advertising for the document required.Preparation of documents as per application & list of documents.Application File.Inspection by the department if required.Liaison department.License issued.Yearly compliance if applicable.Renewal when due.
Why is FDA approval needed
FDA Approval: What it means. FDA approval of a drug means that data on the drug's effects have been reviewed by CDER, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population.
Is FDA approval needed
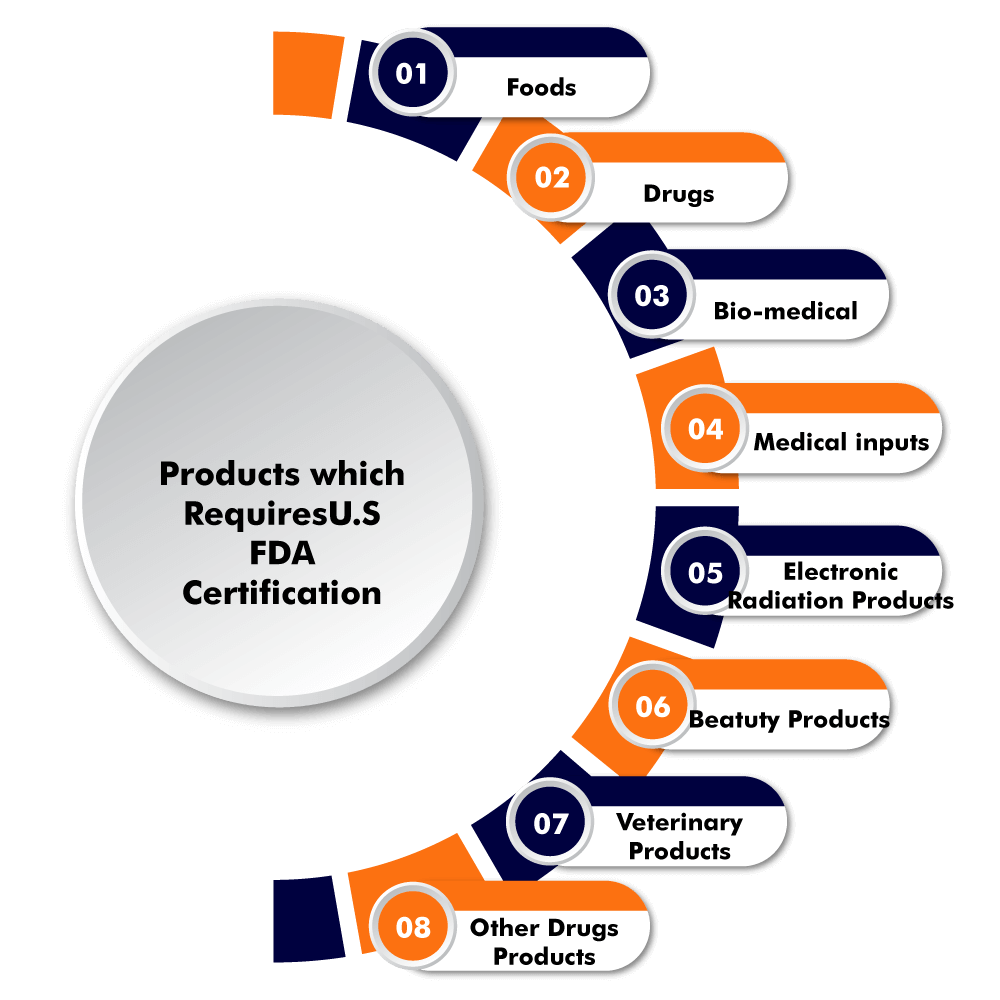
Products requiring FDA premarket approval:
Drugs and biologics are required to be proven safe and effective. According to the FDA, the product's benefits must outweigh any risks related to its intended use. Animal drugs and food additives in animal food, which includes pets, poultry, and livestock. Medical devices.
Who needs FDA approval
Facilities that manufacture, process, pack, or hold food that is intended for human or animal consumption in the United States must register with FDA before beginning these activities.
Why is the FDA important in India
One of the reasons India is under the US FDA lens is that it's the world's largest exporter of generic drugs, making 40% of all new generic drugs that received the FDA's approval last year.
Who needs drug license in India
Drug License in India. In India, the business entity who works in the health care sector such as medicines, drugs or cosmetics shall obtain the Drug License from the appropriate authority. As per the Drugs and Cosmetics Act, License is issued by State/Central Drug Standard Control Organization.
How drugs are regulated in India
The DC Act grants the central government with the power to regulate import, manufacture and sale of drugs and cosmetics including but not limited to defining misbranded, adulterated, spurious and other standards of quality and making rules for their regulation.
What products do not need FDA approval
Under U.S. law, cosmetic products and ingredients do not need FDA approval before they go on the market. The one exception is color additives (other than coloring materials used in coal-tar hair dyes), which must be approved for their intended use.
What type of products require FDA approval
Which Products Need FDA ApprovalHuman and animal drugs.Medical biologics.Medical devices.Food (including animal food)Tobacco products.Cosmetics.Electronic products that emit radiation.
Who needs FDA certification
Products requiring FDA premarket approval:
Drugs and biologics are required to be proven safe and effective. According to the FDA, the product's benefits must outweigh any risks related to its intended use. Animal drugs and food additives in animal food, which includes pets, poultry, and livestock. Medical devices.
Who needs FDA
Domestic and foreign establishments that manufacture, repack, or re-label drug products in the United States are required to register with the FDA. Domestic and foreign drug manufacturers, repackers or re-labelers are also required to list all of their commercially marketed drug products.
Does everything need to be approved by FDA
How to get FDA approval depends on the type of product you are marketing in the United States. FDA does not require FDA approval for all types of products.
Do all drugs need to be FDA approved
FDA Approval is Required by Law
Federal law requires all new drugs in the U.S. be shown to be safe and effective for their intended use prior to marketing. However, some drugs are available in the U.S. even though they have never received the required FDA approval.
Who certifies drugs in India
Under the Drugs and Cosmetics Act, CDSCO is responsible for approval of Drugs, Conduct of Clinical Trials, laying down the standards for Drugs, control over the quality of imported Drugs in the country and coordination of the activities of State Drug Control Organizations by providing expert advice with a view of bring …
Which drug is imported in India without license
Medicine and Drugs Allowed for Import Without a Licence
| Ayurvedic/ Unani name of the crude drugs inclusive of parts | Scientific and/or English name of the drug |
|---|---|
| PLANT ORIGIN DURGS | |
| Shilajit | Black Bitumen, Mineral Pitch Bitumen. |
| Boora-e-Armani | Bole Amreniac, Amrenian Bole. |
| Hajr-ul-Yahud | Fossil Enerinate. |
Who approves drug in India
Under the Drugs and Cosmetics Act, CDSCO is responsible for approval of Drugs, Conduct of Clinical Trials, laying down the standards for Drugs, control over the quality of imported Drugs in the country and coordination of the activities of State Drug Control Organizations by providing expert advice with a view of bring …
Who regulates medicines in India
The Central Drugs Standard Control Organization (CDSCO) is the Central Drug Authority for discharging functions assigned to the Central Government under the Drugs and Cosmetics Act. CDSCO has six zonal offices, four sub-zonal offices, 13 port offices, and seven laboratories under its control.
Do all products have to be FDA approved
While not all products require pre-approval by the FDA, the FDA still has regulatory authority in the event of public safety concerns. The FDA has regulatory authority in the event of a public safety issue related to your product.
What type of products need FDA approval
FDA does not develop or test products itself. The Agency does this pre-market review for new human drugs and biologics (such as vaccines, blood products, biotechnology products and gene therapy), complex medical devices, food and color additives, infant formulas, and animal drugs.