Does ice have heat
Ice is cold, but it still contains some heat energy. An iceberg is made up of particles of water, held in a rigid crystal structure. They still vibrate slightly.
Which has more latent heat ice or water
Thus liquid water has more latent heat than the ice at the same temperature.
What is the energy needed to freeze water
The latent heat of fusion (Lf) is the amount of energy required to change one unit of the sample from liquid to solid. In other words, it is the amount of energy required to freeze the sample. The latent heat of fusion of water is 3.33 x 10 5 J / k g at 0 degrees Celsius.
At what temperature does water convert to ice
32°F
Fresh water transitions between the solid and liquid states at 32°F (0°C) at sea level. At temperatures below 32°F (0°C), liquid water freezes; 32°F (0°C) is the freezing point of water.
What is the heat of fusion of ice
333.5 INTERNATIONAL JOULES PER GRAM with an estimated uncertainty of 0.2 into j/g.
Is Hot ice real ice
Did you Know Sodium acetate or hot ice is an amazing chemical you can prepare yourself from baking soda and vinegar. You can cool a solution of sodium acetate below its melting point and then cause the liquid to crystallize. The crystallization is an exothermic process, so the resulting ice is hot.
Is ice melting latent heat
A total of 334 J of energy are required to melt 1 g of ice at 0°C, which is called the latent heat of melting. At 0°C, liquid water has 334 J g−1 more energy than ice at the same temperature.
Does ice have a high latent heat of fusion
ice has a very high latent heat of fusion.
Is freezing a form of energy
Freezing is an exothermic process, which means it emits energy when it takes place. Freezing is a physical change, which means it is reversible and does not produce new chemicals. As energy is removed from the liquid it freezes and the temperature of the substance stays the same.
Which takes more energy melting or freezing
A total of 334 J of energy are required to melt 1 g of ice at 0°C, which is called the latent heat of melting. At 0°C, liquid water has 334 J g−1 more energy than ice at the same temperature. This energy is released when the liquid water subsequently freezes, and it is called the latent heat of fusion.
Can we convert water into ice
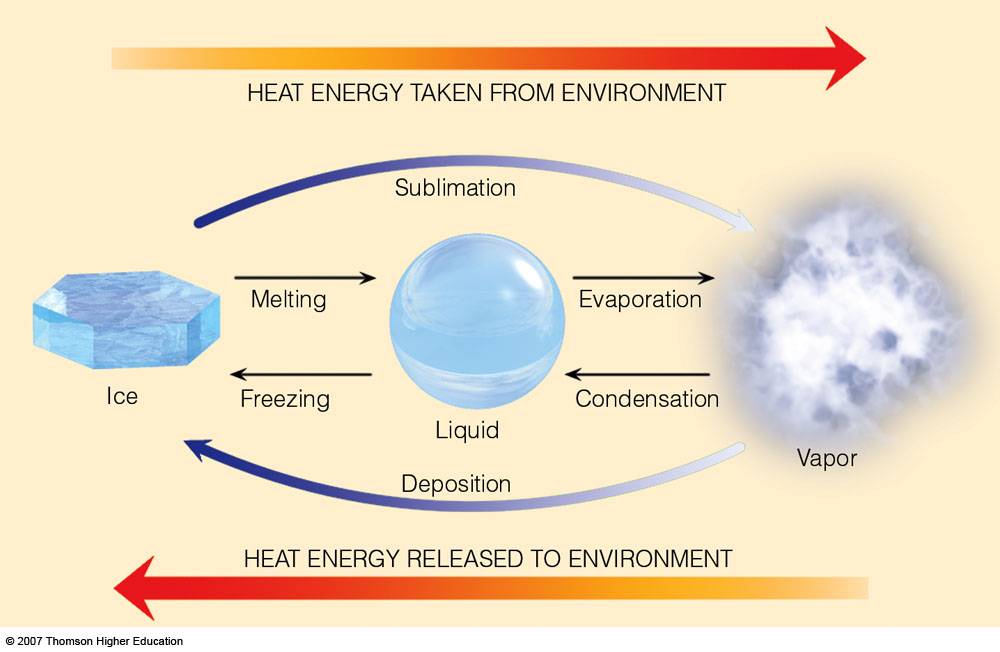
Water can exist as a solid (ice), liquid (water) or gas (vapour or gas). Adding heat can cause ice (a solid) to melt to form water (a liquid). Removing heat causes water (a liquid) to freeze to form ice (a solid).
Can water and ice exist at the same temperature
Solid ice and liquid water coexist at melting point of ice, or freezing point of water (both are same), which is 0 degree Celsius or 273 K. Q. The temperature at which solid and liquid coexist in equilibrium is called ___.
Why melting of ice is called fusion
Answer and Explanation: This phenomenon is called fusion because when two separate solid objects made from the same substance are melted, they can get mixed together into a new one (they fuse).
Is melting and fusion the same thing
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point.
Is dry ice real ice
Dry Ice is frozen carbon dioxide. Unlike most solids, it does not melt into a liquid, but instead changes directly into a gas. This process is called sublimation.
Is Hot Ice Cream real
Unlike traditional ice cream, hot ice cream is made with methylcellulose, which is a thickener that works at high cooking temperatures. Hot ice cream is poached, which also differs from the way conventional ice cream gets made. Whereas regular ice cream melts as it gets warm, hot ice cream melts when it cools down.
Is ice melting sensible or latent
latent heat
In figure 1, very cold ice has heat added to it. The temperature goes up, so that's sensible heat, but once it starts melting, that heat is latent heat (and is represented by the flat parts of the line, during melting or evaporation). Figure 1.
Is melting latent heat of fusion
The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; that associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of vaporization.
What is ice heat of fusion
For water at its normal freezing point of 0 ºC, the specific heat of Fusion is 334 J g-1. This means that to convert 1 g of ice at 0 ºC to 1 g of water at 0 ºC, 334 J of heat must be absorbed by the water.
Is ice melting heat energy
A total of 334 J of energy are required to melt 1 g of ice at 0°C, which is called the latent heat of melting. At 0°C, liquid water has 334 J g−1 more energy than ice at the same temperature. This energy is released when the liquid water subsequently freezes, and it is called the latent heat of fusion.
Does freezing create heat
Freezing is almost always an exothermic process, meaning that as liquid changes into solid, heat and pressure are released. This is often seen as counter-intuitive, since the temperature of the material does not rise during freezing, except if the liquid were supercooled.
Does ice lose energy when melting
To melt, ice energy is required. Heat is the form of energy supplied. On being heated the ice starts to melt because heat breaks the bond between the molecules and then the molecules are free to move apart. Thus, ice molecule gains energy and begin to move freely.
Is freezing losing energy
The change from the liquid state to the solid state is called freezing. As the liquid cools, it loses thermal energy. As a result, its particles slow down and come closer together. Attractive forces begin to trap particles, and the crystals of a solid begin to form.
Can water melt ice
Why Ice Melts at Different Rates in Air and Water. Assuming the air and water are both the same temperature, ice usually melts more quickly in water. This is because the molecules in water are more tightly packed than the molecules in the air, allowing more contact with the ice and a greater rate of heat transfer.
Can ice turn into steam
Sublimation is the conversion between the solid and the gaseous phases of matter, with no intermediate liquid stage. For those of us interested in the water cycle, sublimation is most often used to describe the process of snow and ice changing into water vapor (gas) in the air without first melting into water.


